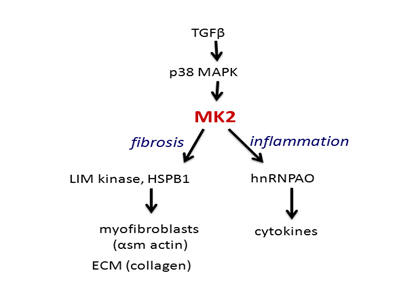
MK2 in Fibrosis
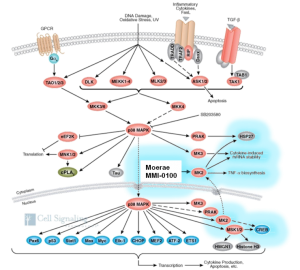
The transforming growth factor beta (TGF-β)/p38 pathway regulates critical components of fibrogenesis and as such, has become a focus of anti-fibrotic drug discovery. Pirfenidone (Esbriet®, InterMune, currently registered in Europe and Japan), the only approved therapy for idiopathic pulmonary fibrosis, has been suggested to target this pathway. Several other IPF agents in development purportedly target the TGF-β/p38 pathway at various points as well. However, as TGF-β also plays an essential role in host defense and cancer immunosurveillance, the pleitropic activity of the pathway poses a challenge for developing highly specific anti-fibrotic agents with sufficiently low off-target toxicity and adequate therapeutic ratio.
Given this challenge, MK2, a terminal kinase in the p38 pathway has emerged as a target of interest in fibrosis. This downstream targeting enables greater specificity of action, lower off-target signal amplification, and potentially reduced toxicity versus other TGF-β/p38-directed agents.
However, MK2 historically has been challenging as a druggable target: its intracellular location precludes an antibody approach, and the high degree of sequence identity with other closely related kinases at the ATP binding site has hampered development of a truly selective small molecule inhibitor.
THE MOERAE MATRIX APPROACH
Moerae Matrix has overcome these challenges by designing small, intracellulary directed peptides that target MK2 at the substrate binding domain, a region of high evolutionary conservation across species and low sequence identity across the kinase family. With a high degree of target specificity, low molecular weight, and excellent in vivo potency, Moerae Matrix peptides have excellent pharmacologic and pharmaceutical properties.