MMI-0100
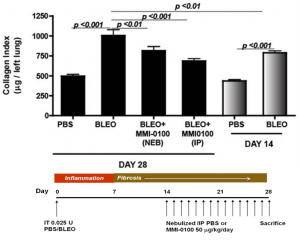
MMI-0100 demonstrates significant reduction in collagen deposition even when dosing is initiated seven days after onset of fibrosis in bleomycin mouse model
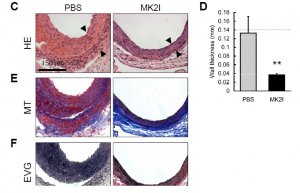
Moerae Matrix’s first development candidate, MMI-0100, is in preclinical development for treatment of idiopathic pulmonary fibrosis and other acute fibrotic indications. In the bleomycin mouse model, the industry standard model of pulmonary fibrosis, many agents are able to demonstrate anti-fibrotic activity when dosed prophylactically, prior to the onset of fibrosis. However, MMI-0100 has the unique ability to show a therapeutic benefit by abrogating collagen deposition when dosed after the onset of fibrosis.
MMI-0100’s anti-fibrotic activity is consistent in models of multiple fibrotic conditions including vascular intimal hyperplasia, post-surgical adhesions, and cutaneous scarring. Across model systems, MMI-0100 demonstrates excellent in vivo potency. The company has compiled a full IND-enabling data package on MMI-0100; the compound also demonstrates a favorable toxicology profile and highly scalable manufacturing process. In support of chronic administration for treatment of IPF, the company is developing a pulmonary formulation of MMI-0100 for dry powder inhalation delivery.